INTRODUCTION
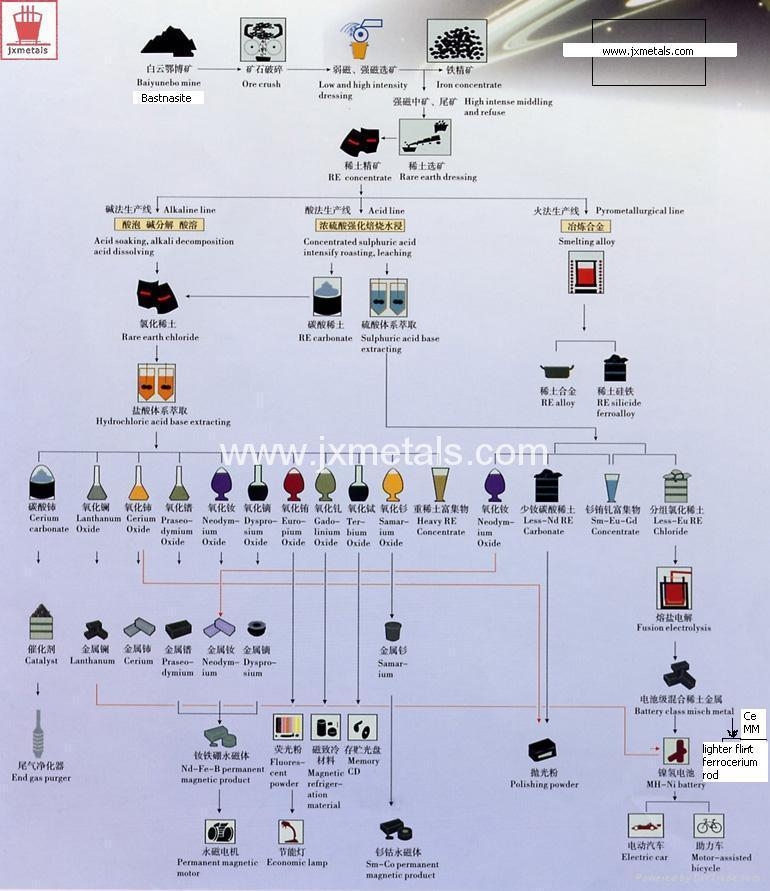
When a lanthanide mineral is processed without separation into the individual lanthanides to yield a metal termed Mischmetal, (see the process flow chart on the left illustration).
Mischmetal was originated from German: Mischmetall - "mixed metals" of rare earth, it is an alloy of rare earth elements in the "natural-ratio" as they occur in the ore body. it is often usual to extract the small amount of heavy lanthanides or of high value from the precursor before metal production, consequently mischmetal contains predominantly the first four lanthanides(Cerium, Lanthanum, Praseodymium, Neodymium) of the light Ln's (Lanthanide). A typical composition includes approximately 60% cerium and 30% lanthanum, with small amounts of neodymium and praseodymium detailed as below:
| Chemical Composition | Typical | Guaranteed | |||
| Total Rare Earth Metals | (TREM) 99.02% | 98.50-99.50% | |||
| Ce/REM | 68.70% | 62.00%min | |||
| La/REM | 30.88% | 30.00%min | |||
| Pr/REM | 0.31% | 1.0%max | |||
| Nd/REM | 0.30% | 1.0%max | |||
| Sm/REM | <0.10% | 0.1%max | |||
| Eu/REM | <0.10% | 0.1%max | |||
| Gd/REM | <0.10% | 0.1%max | |||
| Y /REM | <0.10% | 0.1%max | |||
| Fe | 0.49% | 1.0%max | |||
| Si | 0.03% | 0.2%max | |||
| S | 0.02% | 0.04%max | |||
| P | <0.02% | 0.02%max | |||

The trade names for mischmetal are sometime called as follows :
| mischmetal, 28053010 | Lanthanum rich mischmetal | ||||||
| Cerio Mischmetal | CeMM | ||||||
| MISCH metal | Mischmetall | ||||||
| Misch Metal | mishmetal | ||||||
| Less-Mg Misch Metal | Re mischmetal | ||||||
| Rare Earth/misch metal | Cerium(Mischmetal) | ||||||
| Cerium Misch Metal | CER-mischmetall | ||||||
| Cerium (Misch) Metal | Cerium Mischmetall | ||||||
| Cerio-Mischmetal | Ce-mischmetall | ||||||
| Ce, mischmetal | Cer MM | ||||||
| Ce. Mischmetal | Mm=Mischmetall | ||||||
| Ce Mischmetal alloys | Ce/Fe-Mischmetall | ||||||
| MM | El Mischmetal | ||||||
| Mg-Zn-Misch metal alloys | Cerium rich Mischmetal | ||||||
| Cerium Mischmetall | High Cerium Mischmetal | ||||||
| Mischmetall | Rare earth metals ( Mischmetal ) | ||||||
| CER-Mischmetall | rare earth metals/mischmetal | ||||||
| CerMM | Ce-rich mischmetal | ||||||
| Cer Mischmetal | La-rich mischmetal | ||||||
| Cerium-rich mischmetal | El Mischmetal es una aleación | ||||||
| Ce-Mischmetall | Cerium MM | ||||||
| Cereisen | Cerium mishmetal | ||||||
| Mischmetal(Rare Earths) | Mixed rare earth metals | ||||||
| michmetal | Misch metal (sometimes called Cereisen in German) | ||||||
| Ln Mischmetal 50% Cerium | Ce Mischmetal (alloying, deoxidizing and desulphurizing agent) | ||||||
| Lanthanide mischmetal | lanthanide (rare earth) metals | ||||||
| mixed metals | pyrophoric alloy | ||||||
| rare earth mischmetal | mischmetal alloy | ||||||
| High Nd mischmetal | Mischmetal (lanthanide-iron-silicon) | ||||||
| praseodimio | Lanthaan | ||||||
| néodyme | lanthane | ||||||
| Neodym | Lanthan | ||||||
| neodimio | lantanio | ||||||
| praséodyme | lantano | ||||||
| Praseodym | Lantan | ||||||
REE = Rare Earth Elements, lanthanum to lutetium by atomic weight plus yttrium
TREM =Total Rare Earth Metals
TREO =Total Rare Earth elements in Oxide, calculated as oxides, including lanthanum to lutetium plus yttrium
LREE = Light Rare Earth Elements, lanthanum to samarium by atomic weight
HREE = Heavy Rare Earth Elements, eur to lutetium plus yttrium
LREO = Light Rare Earth Elements in Oxide, as per LREE above, calculated as oxides
HREO = Heavy Rare Earth elements in Oxide, as per HREE above, calculated as oxides

To provide an excellent firestarter in the survival kits, we use our Cerium Mischmetal and Iron, Magnesium & other metals to make Ferrocerium rod, Ferrocerium is the "flint" in lighters, and its ability to give a large number of sparks when scraped against a rough surface (pyrophoricity) is used in many other applications, such as the firestarter, camping fire, outdoor survival firestarting, etc.,
While ferrocerium-and-steel function in a similar way to flint-and-steel in fire starting, ferrocerium actually takes on the role that steel played in traditional methods. When small shavings of it are removed quickly enough, the heat generated by friction is enough to ignite those shavings. The sparks generated are in fact tiny pieces of burning metal. FerroCerium flint rod equipped with high carbon steel scraper, then produced fireesteel, it is usually well-known as the Swedish firesteel, which was originally developed for the Swedish Department of Defense.
Swedish Fire Steel is truly "a flash of genius". Its 3,000ºC (5,000ºF) spark makes fire building easy in any weather, at any altitude. it has been approved by the International Survival Instructors Association, and it is used by a number of armies around the world. The Swedish firesteel is a very useful fire starter in scouting and camping, sparking entertainment, clockwork toys, strikers for welding torches, etc., it is the best compact firestarter we can easily use, plus its dependability,made it a favorite of survival experts, hunters, fishermen and campers. It works equally well when wet or dry. Swedish Fire Steel has even found its way into cabins and backyards as a fool-proof way to light stoves and gas-barbecues.
It comes in two sizes: the larger Army Model (12,000 strikes) is ideal for camping and conventional survival kits and the Scout Model (3,000 strikes) is just barely small enough to carry on a key ring.

This emergency fire starters come under many different trade names such as artificial flint rods, Auer metal, Blastmatch, Blastmatch firesteel, blast match, Camping flints, cer/iron, Cerium flint, Cerium Mischmetal striking flint, Cerium alloy, Cerium alloys, cerium iron rods, cerium iron rod, Camp Fire Starter, Ferrocerium, Ferro-Cerium, Ferrocerium based fire-starter, Ferrocerium firestarter, Ferro mischmetal, ferocium, Ferrocerium rod, ferrocerium rods, Ferrocerium sticks, Ferro Cerium rod, ferrocium rods, ferro rod, ferro rods, Fire-Flint and Steel, Fire Steel, Firesteel, Fire and steel fire starter, Fire & Steel, Firesteels, Fire steels, Fire steel striker, Flint firestarter, Flint fire starter, Firestarter sticks, flint fire-starting, fire rod, fire bar, fire stone, firestone, firestrikers, fire strikers, fire striker, fire starter rods, firesteel rod, firesteel rods, firesteel sticks, firesteel stick, Flint, flints, Flint rod, flint rods, flint sticks, flint bar, Flint fire starter, flint and steel bushcraft fire lighters, flint striker, Flint & steel, Flint and steel, flint n' steel, Flints & Steels, Flints and Steels, Flintstone, flint stone, FC rods, iron rod, iron rods, Lighter flints, lighter flint, lighter flint misch metal (mesch metal), Magnesium-Ferrocerium Firestarter, Magnesium Ferrocerium Fire Starter, Magnesium Ferro Cerium Fire Starter, Magnesium campfire fire starter, Metal-Match, Metal-Matches, Metal match, Misch metal rods, Blast Match Fire Starter, Blast Match Flint Fire Starter, mischmetal rod, mischmetal rods, misch metal rod, mischmetal flints, mischmetal flint, pyrophoric alloys, Sparking rod, Sparking flint rods, Survival flints, survival fire starters, Swedish Army Firesteel, Camping Ultimate Survival Blast Match, etc.,
The Application of Rare Earth Metals Widening
Despite Lack of Engineering Data.
By Roman Lundin and John R. Wilson
Abstract
Uses of the pure rare earth metals or of alloys containing them as a dominant component have understandably been limited by their high cost, extreme sensitivity to contaminants, poor mechanical properties, high chemical reactivity and, most significantly, by a broad lack of physical property data and metallurgical information on these materials. Nevertheless, they are finding increasing uses as low-level but very important alloying additives for stainless steels and for aluminum alloys, silicon alloys and magnesium alloys. In addition to their historical use in spark igniters (e.g., lighter flints or ferrocerium rods) and the so-called "supermagnet" alloys such as gadolinium-cobalt, the rare earths are also finding new uses in superconducting devices, lithium/metal hydride batteries and a wide variety of ceramic and other materials. This paper reviews what is known of the physical and chemical characteristics of these unusual materials and discusses many of their current and potential future commercial uses.
Group 1 lanthanides have low melting points and high boiling points -lanthanum, cerium, praseodymium, neodymium.
Group 2 lanthanides have high melting points and high boiling points - gadolinium, terbium, yttrium, lutetium.
Group 3 lanthanides have high melting points, mid to low boiling points and a high vapor pressure at the melting point - dysprosium, holmium, erbium and scandium.
Group 4 lanthanides have low boiling points - samarium, eur , ytterbium and thulium.
Group 5 lanthanides - this group contains just one element, promethium, which really belongs in Group 1 but is highly radioactive and for that reason has no significant commercial uses.
The grouping of the lanthanides in this way also correlates with their processing characteristics. For example, the group 3 lanthanides are difficult to handle in vacuum-remelting equipment because of their high vapor pressures - they are better refined by a sublimation method. The group 2 and group 3 lanthanides are difficult to contain in metal or ceramic crucibles as liquids at high temperature because of their high melting points. The group 1 lanthanides offer some of the broadest liquid ranges (LR = BP-MP) of any of the elements. Note that the group 4 lanthanides do not have unusually high vapor pressures close to the melting point despite their relatively low boiling temperatures.
Despite the differences that permit this classification, the rare earths are chemically more similar than different, a characteristic that has presented major difficulties in their separation and purification.

2. Established Rare Earth Metallurgical Uses
Rare earths have experienced gradual growth in commercial use since their discovery. Misch metal (generally referred to as Mm), a relatively impure alloy of cerium and lanthanum with other rare earth elements that is the direct result of refining RE mineral concentrates without separation of the individual elements, has been used as a flint material in lighters and firearms for many years. Both cerium and lanthanum are pyrophoric and, as a result, small particles of the alloy ignite in air when struck off the flint. Fortunately, the mischmetal usually contains a high level of both iron and interstitial elements, which make it brittle and easily able to form sparks. Mischmetal has other uses, too. Interstitial and iron-free Mm is being evaluated by a number of researchers as a lower-cost substitute for pure rare earth metals in applications where the presence of other rare earths is non-critical. The problems in applying mischmetal, however, derive from the characteristics that make it effective in flints - the material is typically embrittled by a high interstitial content and by about 5-10 wt % iron (the latter forms numerous intermetallic compounds with the rare earth metals). In addition, mischmetal varies widely in composition according to source; it may be cerium-rich mischmetal, lanthanum-rich mischmetal and may contain more or less of Nd, Pr, Sm and several other rare earths. Historically, no attempt has been made to remove the interstitials from mischmetal, which may contain several wt % of these impurities.
2.1 Improving the properties of irons and steels
Rare earth elements, most notably cerium and mischmetal, have also been used as minor alloying additives for controlling inclusions in cast irons and steels. The cerium appears to combine with the sulfide inclusions that are invariably present in these materials to form particles with a more rounded morphology that is less likely to promote cracking. The normal flake-like morphology of graphite particles in nodular irons may also be modified to a spheroidal form that promotes greater ductility. Both results may be due partly to the effect of Ce in modifying the surface properties of the metal or sulfide. The extreme chemical affinity of the rare earth elements for almost anything that they may contact suggests that they will also interact strongly with inclusions in most metals, but little is known about the mechanism by which this occurs.
A more recent discovery suggests that small additions of the lanthanides may confer even greater protection on those metals and alloys that are already well protected from corrosion by oxide films. These include the iron-chromium and iron-chromium-nickel stainless steels (i.e., both the ferritic and austenitic alloys), and most other alloys that are dependent on chromium for their corrosion/oxidation resistance. Further study may suggest that the effect is even broader - e.g., that the protection provided by all spinel-forming oxides such as Al2O3 or Cr2O3 is enhanced in this way. Use of rare earths as alloying additions for corrosion control shows promise of becoming a major growth market, for example, Mischmetal can also be used as the alloying additive of a Zinc-Aluminium alloy named Zinc-5 % Aluminum-Mischmetal (Zn-5Al-MM) alloy, having a 95% Zn-5% Al-trace mischmetal (cerium, lanthanum), which is often used to be as the coatings fore steel, to enhance the product life for certain applications.
There is also strong evidence that at least cerium acts as a grain-refining agent in some steel compositions, just as it apparently does for aluminum and magnesium alloys (see below), with corresponding improvements in mechanical properties and fatigue resistance.
2.2 Improving the properties of Non-Ferrous Metals and Alloys
High-Strength Aluminum Alloys
Lanthanide additions have been very effective in enhancing the mechanical properties (UTS, impact) and reducing the notch-sensitivity and increasing the fatigue life of a range of high-strength aluminum alloys, most notably the Al-Li alloys most commonly used for airframe construction. Good results have also been obtained for Al-Fe-V-Si alloys and more recently for the high-silicon Al-Si alloys used for (e.g.) cylinder liners. Corrosion resistance and hence resistance to stress-corrosion cracking is also greatly improved. Cerium or mischmetal are the most commonly-used additives (the amounts required are small, typically much less than 1 wt.%). The mechanism by which the lanthanides achieve this effect is not completely clear. Grain refinement is evident and it appears likely that the morphology of many of the intermetallics present is shifted from platelike toward spherical in shape, which reduces their impact as crack initiators. The effect on the corrosion resistance of these alloys is likely to be due to the effect of cerium (or other lanthanide) oxide on the protective capabilities of Al2O3, but this has not yet been shown, nor has the mechanism been studied.
Much of the recent work on the metallurgical uses of the lanthanides has been reported from
Amorphous Alloys
Recently, as a result of work at AlliedSignal and elsewhere, interest has been growing in the production and use of amorphous aluminum, magnesium and other alloys. These are typically produced by rapid quenching of the molten alloy onto a cooled surface. The amorphous or "glassy" metals have a number of very desirable properties including, for example, unusually high corrosion resistance, thought to be due to the lack of surface/grain boundary intersects, but they are somewhat thermally unstable and will recrystallize to a more normal crystalline morphology. The addition of small amounts of rare earths, usually accompanied by transition metals, to the melt prior to 'casting' results in a material that, with carefully-controlled annealing, produces a nanocrystalline (i.e., extremely finely crystalline) structure that is more stable than the amorphous material but has better mechanical properties and equally good corrosion resistance (over-annealing to a more coarse structure offsets these advantages). New uses are rapidly developing for these materials. Once again, the mechanism by which the lanthanide additions exert their effect is not yet known. Typically, addition amounts are less than 1 wt.%, but precise control of both chemistry and processing is necessary.
Galvanizing Applications
Mischmetal is also used very effectively in an improved zinc galvanizing product called Galfan™ (developed by ILZRO/Weirton Steel). This is a zinc-5 wt.% aluminum alloy with small Mm additions that is used as a substitute for 'straight' zinc. It has been shown to be very effective in most galvanize applications with the possible exception of heavily-contaminated industrial environments. It is in extensive use in
Mischmetal or pure rare earth additions are also surprisingly beneficial in magnesium and aluminum-magnesium cast alloys. Once again, the grain structure is refined, the negative impact of intermetallics on notch sensitivity, toughness and strength is offset and corrosion resistance greatly improved. In some cases (e.g., Al8Mg5), formation of the intermetallic may be suppressed.
The beneficial effects of the lanthanides on the non-ferrous metals extends, not surprisingly, to their metal-matrix composites. Once again, the presence of the rare earths results in grain refinement, improved mechanical properties and, apparently, improved intermetallic morphology in addition to enhanced corrosion resistance.
Numerous other claims for benefits have been made, mostly in the Chinese, and occasionally in the Russian, literature. In most cases, these claims have not yet been substantiated by Western research or practice but there is no reason to doubt their validity, based on the reliability of the work discussed earlier.
2.3 Nuclear Applications of Rare Earth Metals
Eur , gadolinium and dysprosium have large capture cross-sections for thermal neutrons and thus are often incorporated into control rods to regulate reactor operation. Rare earth elements can also be used as burnable neutron absorbers to maintain the reactor flux at a more constant level. Obviously, with the relative demise of the nuclear power industry, this is a small and non-growing market for the rare earth metals.
2.4 Supermagnets and Superconductors
Although not the theme of this article, one of the most important applications of the rare earth elements is in "supermagnet" materials - usually permanent magnets based on gadolinium-cobalt, samarium-cobalt or neodymium-iron-boron with other metals in minor amounts. The cobalt alloys offer the highest permanent magnet performance known. Use of these materials continues to grow rapidly at more than 15% annually as power generating devices in automobiles and aircraft grow smaller and more compact while offering higher capacities. Tight control of purity is required to achieve optimum performance.
A number of intermetallic compounds and oxides containing rare earth metals are being evaluated as so-called high TC superconductors - materials that demonstrate superconductivity (vanishingly low electrical resistivity) at temperatures well above 0 deg. K (absolute zero). Once academic curiosities, these materials now offer considerable promise for use in future industrial high-current devices and generating equipment. Development work in this area appears to be growing exponentially and excellent progress is being made. Once again, purity seems to important in achieving optimum performance.
2.5 Other Metallurgical Applications
While there are many possible metallurgical applications for the RE metals, the high cost of these metals usually results in an alternative choice being made. However, there are some areas, such as those mentioned in sections 5.1 and 5.2, that make use of the unique characteristics of the rare earths. These include uses in thermite devices and in tracer ammunition, both of which utilize the pyrophoric characteristics of these metals to good effect. The high cost of the rare earth metals, even mischmetal, makes it unlikely that they will replace aluminum or (occasionally) magnesium in conventional thermite mixtures for steel rail welding and equivalent uses. However, in view of the favorable effect of rare earth elements on the performance of cast steels (see above), there may be an opportunity to use rare earths together with aluminum or magnesium in sophisticated thermite mixtures for welding applications in which a high performance weld is required. They could be especially valuable for underwater thermite welding systems.
Pyrophoric formulations are also used in a variety of weapons systems (mischmetal has featured in these for many years) such as tracer shells and incendiary weapons of various kinds.
The writers are frequently asked to provide fabricated rare earth samples (wire, tube, sheet) for other unspecified applications. Clearly, other uses for pure rare earth metals are also being investigated.
2.6 Other Uses of the Rare Earth Elements and their Compounds
While this article has intentionally focused on the larger-scale metallurgical uses of the rare earth metals, there are very many uses of these elements that fall into other categories. The total markets for cerium and lanthanum are large since the oxides and other compounds of these metals have found broad industrial application in products that range from camera lenses to polishing compounds. Some of these are still evolving as the technology is improved. For example (and this list is far from comprehensive):
¨ Aluminum-scandium alloys are finding uses in sporting goods such as golf clubs (along with almost any other combination of metals in this fashion-conscious sport!), baseball bats, bike frames, etc.
¨ A wide range of specialty ceramics is emerging, based on rare earth oxides. A comprehensive discussion would require a major publication of its own! Yttria (Y2O3) has long been used to stabilize zirconia (ZrO2) ceramics at high temperature but recent research has uncovered numerous additional applications for ceramics containing rare earth oxides. For example, a "second generation" family of high TC superconductors such as YBa2Cu3O7 has appeared which, while very difficult to fabricate into conductors (such as wires) offer some exciting properties. Much of the early work on high-TC oxide superconductors focused on the so-called ABO3 perovskites such as LaTiO3 and later on layered perovskites such as (La,Sr)2CuO4. Rare earths are also commonly found in solid-state zirconia-based solid electrolytes used in commercial oxygen sensors (e.g., for automotive use) and in laboratory applications. Rare earth nitride ceramics are also of interest.
¨ The rare earth oxides find wide use as components of catalysts used in chemical processing (for oxidation, amidoxidation, polymerization) and in the treatment of exhaust gases produced by internal combustion engines. They are also to be found in silicone stabilization additive packages, diesel fuel additives (for particulates control) and in corrosion inhibitors.
¨ Heated rare earth metals are used as scavengers or "getters" for oxygen and nitrogen, as well as other gases such as hydrogen, in high-vacuum systems and in nuclear applications (especially those using liquid metal coolants which are highly corrosive in the presence of even minute amounts of oxygen). Hafnium and zirconium also serve well in this application.
¨ Recent work at the DOE Ames Lab and elsewhere has shown the potential of using tunable magnetic regenerator intermetallics such as Gd5(Ge2Si2), which exhibit so-called 'giant magnetocaloric effects', as the basis for magnetic refrigeration systems.
¨ Neodymium, scandium and lanthanum are used in sodium discharge lamps to moderate the yellow coloration of the light generated by these. Rare earth oxides were also used for many years to coat filaments in vacuum tubes, but this market has almost disappeared.
¨ Radioactive yttrium and terbium wire are used in medicine as implants for cancer therapy (e.g., in the so-called 'gamma-wire' therapy).
¨ Several of the rare earths - lanthanum, praseodymium, for example, are being used in 'new generation' long-life metal/hydride batteries.
¨ Chinese workers claim that certain of the RE metals are useful in agriculture, perhaps as plant growth stimulators. This has not yet been confirmed by European or
2.7 Toxicology of the rare Earths
There appears to have been little systematic work on the toxicology of the rare earth metals and their compounds. Insufficient data is available even to complete MSDS sheets on most shipments of RE products. The pure metals (primarily Y and Tb) have seen limited use a medical implant materials and the literature has general references to the metals as benign and harmless but this does not seem to be completely in keeping with the known reactivity of these metals. More work is needed in this area. Meanwhile, the RE metals and compounds should be handled circumspectly.
3. Future New Uses for Rare Earths
3.1 Pure Rare Earth Metals or Alloys
Extraction and Refining
Better definition of the mechanical and physical properties of the rare earth metals might eventually lead to additional uses of these metals in pure or alloyed form, but only if lower-cost methods of extraction (from the mineral concentrates, which are relatively inexpensive) and purification of the metals can be developed. RE compounds such as the oxides are available in moderate purity, although even these require extensive and repetitive ion exchange processing (as soluble salts) before they are sufficiently well separated from compounds of other rare earths. The oxides are then easily produced at acceptable purity levels (e.g., 5N or better). Arris International supplies 6N oxides for most rare earth metals. However, although there is a demand for ultra-high purity (e.g., 7N) oxides, these are not readily available.
Metallothermic reduction of a suitable compound (such as the RE chloride or, more often, fluoride) using 10% excess calcium in a helium or argon atmosphere can then be used to produce a relatively crude metal that is loaded with both halide atoms, calcium and often oxygen. The resulting metal is extremely impure and therefore brittle. Mischmetal is produced in this way from the unseparated oxides or halides, but is seldom further refined.
The high purity metal that is required for research purposes and for the few commercial uses for which the single RE metals are needed can then be obtained by one of several methods:
¨ Vacuum remelting at very high vacuum, usually in a tantalum crucible (many of the more serious impurities evaporate); several techniques are used depending on the vapor pressure of the metal being refined. The high-melting, high-VP metals such as thulium can be purified by sublimation.
¨ Floating-zone refining, again in high vacuum. This has much the same effect but results in a more pure product and, when fully developed (which is far from easy!) is less costly.
¨ Electrochemical refining using a molten fluoride or chloride electrolyte with the crude metal as the cathode (it can be solid and contained in a titanium or tantalum basket) and a carbon anode. Very high purity metal can be produced in this way with little or no re-contamination of the metal with fluorine or chlorine provided that the refining potential remains 'on' while the cell is cooled. It remains to be seen whether this technology, which borrows heavily from aluminum and magnesium production and refining, can be scaled up economically.
¨ It is also possible that the rare earths could be all of extracted (from the oxides or halides), separated and purified electrochemically in a multi-step process. This is being evaluated by the authors.
The Pure Rare Earths
Once adequate mechanical and physical property data are available and pricing can be projected for production in significant volume (in this context, kilograms rather than grams) decisions can be made regarding potential new applications. However, it is difficult to see which materials could be replaced by any of the pure rare earths. The latter offer none of the advantages of Al, Mg or Ti (low density and excellent oxidation resistance) or steels (strength) and are available only at higher cost. However, they seem to be able to play a very valuable role in improving the properties and therefore extending the service limits of these almost ubiquitous materials. Perhaps in this context, the RE metals will be "always the bridesmaid, never the bride".
"Pure" Mischmetal
One possible exception to this rule may be mischmetal (Mm). Alloys of the rare earth metals generally (but not universally) behave a little like a pure rare earth. Thus mischmetal, which, depending on source, may contain up to 70 wt.% of Ce + La (which reportedly form a continuous series of solid solutions) plus about 20-25 wt.% of other rare earths and 5-10 wt.% iron, may, once the iron and interstitials are removed, offer the performance of a pure rare earth without the high cost of separating the rare earth components. Documentation of the properties of mischmetal in almost any condition of purity are lacking so it is, once again, not yet possible to support this conjecture.
3.2 Rare Earths in Alloys
In their various supporting roles, the future of the rare earth elements looks bright indeed. Recent data on their effectiveness in improving the mechanical performance, fatigue resistance and also corrosion resistance of a growing variety of aluminum-, magnesium-, zinc and iron-based alloys, including the stainless steels, are very persuasive. The only possible downside seems to be the lack of any in-depth understanding of the mechanisms by which these improvements are achieved. Experience suggests that wide commercial use of any new technology without a sound technical foundation can lead to catastrophic results (the use of aluminum alloys in pressurized jet aircraft in the 1950s before an understanding of fatigue mechanisms was developed is one example). Thus Arris International, with others in the rare earth materials industry, will be focusing in the near future on developing a better understanding of the mechanism by which rare earth metals control alloy morphology and facilitate the development of enhanced corrosion resistance in these alloys. In the process, new alloy formulations will undoubtedly result, since this work is at such an early stage.

4. Summary: Bright Future!
Given the quantities involved, it seems likely that the future market for master alloys based on the rare earth metals will be both large and fast-growing. Other promising uses are the high-Tc superconductors, metal-hydride batteries and the permanent magnet market. In this article, we have largely ignored the markets for rare earth compounds, especially the oxides, but these are already very large and growing rapidly. Fortunately, the rare earth minerals are widespread and plentiful and the present supply will take care of any foreseeable demand for many years to come. Technology now under development will result in needed cost reductions for many rare earth products and will promote the emergence of many new markets. The future looks bright!
| MATERIAL SAFETY DATA SHEET | |||||||
| FERROCERIUM FLINT | |||||||
| 1. Identification of Material and the Manufacturer: | |||||||
| Product Name: | FerroCerium Flint rod blank | ||||||
| Synonyms : | Cerium Mischmetal striking flint, Cerium Misch metal sticks, Cerium mischmetal stick, cerium iron rods, cerium iron rod, esstential outdoor survival gear, Ferrocerium, Ferro-Cerium, Ferrocerium based fire-starter, | ||||||
| Ferrocerium firestarter, Ferro mischmetal, Ferrocerium rod, ferrocerium rods, Ferrocerium sticks, Ferro Cerium rod, ferrocium rods, ferro rod, iron rod, ferro rods, iron rods. | |||||||
| Fire-Flint and Steel, Fire Steel, Firesteel, Fire and steel fire starter, Fire & Steel, Firesteels, Fire steels, Fire steel striker, Flint firestarter, Flint fire starter, Firestarter sticks, flint fire-starting, firestrikers, fire strikers, fire striker, | |||||||
| fire starter rods, firesteel rod, firesteel rods, firesteel sticks, firesteel stick, Flint, lighter flint, flints, Flint rod, lighter flints, flint rods, flint sticks, flint bar, Ferrocerium Flint fire starter, Ferrocerium camping & emergency flint fire starters, flint firestarter, flint firestarters, firestarting sparking flint, flint device, flint devices, lighter flint misch metal (mesch metal), flint and steel bushcraft fire lighters, flint striker, Flint & steel, Flint and steel, flint n' steel, | |||||||
| Flints & Steels, Flints and Steels, Magnesium-Ferrocerium Firestarter, Magnesium Ferrocerium Fire Starter, Magnesium Ferro Cerium Fire Starter, Magnesium campfire fire starter, Metal-Match, Metal-Matches, Metal match, Misch metal rods, Ultimate Survival Blast Match, Blast Match Fire Starter, Blast Match Flint Fire Starter, mischmetal rod, mischmetal rods, misch metal rod, mischmetal flints, mischmetal flint, mischmetall stick, | |||||||
| mischmetall sticks, misch metal stick, misch metal sticks, Sparking rod, Sparking flint rods, Sparking pad, Sparking pad, Flint pad, Flint pads, Survival flints, survival fire starters, Swedish Army Firesteel Camping Camp Fire Starter, pyrophoric alloys, "ferrocerium UN1323", iron cerium rod, fc rod, fc rods, high technologies survival tools, ultimate survival tool kits, ferrocerium strikeforce, Sparking Flint, | |||||||
| Sparking Flints, Cerium sparking flint, Cerium sparking flints, sparking alloy, sparking entertainment, flint slab, flint disc, Mischmetal Fire Starter rod, Mischmetal Fire Starter rods, Cerium Mischmetal Fire Starters, Mischmetal firestarter, Cerium Mischmetal firestarters, Flint and steel kits for primitive fire making, flint striker, flint strikers, handless striker, handless strikers, ferrocerium blank, ferrocerium blanks, | |||||||
| ferrocerium rod blank, ferrocerium rod blanks, flint blank, flint blanks, flint rod blank, flint rod blanks, blank flint, blank flints, ferro rod blank, ferro rod blanks, firesteel blank, firesteel blanks, fire steel blank, fire steel blanks, flintsteel blank, flintsteel blanks, flint steel blank, flint steel blanks, Outdoor survival kits in straight rods, Survival kits in rod, pyrophorous alloy, Auer metal, spark metal, ferrocerio. | |||||||
| Identification of the manufacturer, supplier: | |||||||
| Company Identification : SHANGHAI JIANGXI METALS CO., LTD. | |||||||
| Add.: 718 Huamu Road, Pudong New Zone, Shanghai 201204 China | |||||||
| Tel : +86 21 5059 7606 | |||||||
| Fax : +86 21 5059 7608 | |||||||
| E-mail: info@jxmetals.com | |||||||
| www.jxmetals.com SurvivalFlint.com LighterFlint.com | |||||||
| 2. Composition and Information on Ingredients | |||||||
| Composition | Value | CAS # | EINECS# | ||||
| Total Rare | |||||||
| Earth Metal | |||||||
| (TREM) | 87% Min. | ||||||
| Iron (Fe) | 6% - 20% | 7439-89-6 | 2310964 | ||||
| Magnesium(Mg) | 1.1-4.0% | 7439-95-4 | 2311046 | ||||
| Total Rare Earth Metal Distribution | |||||||
| Cerium/TREM | 48-58% | 7440-45-1 | 2311549 | ||||
| Lanthanum/TREM | 25-46% | 7439-91-0 | 2310990 | ||||
| Praseodymium/TREM | 0-7% | 7440-00-8 | 2311093 | ||||
| Neodymium/TREM | 0-3% | 7440-10-0 | 2311203 | ||||
| 3. Hazards Identification | |||||||
| Risks of burning off for fine material or explosions after raising the dust or shavings. | |||||||
| Flash Point and Method Used: | None | ||||||
| Auto-Ignition Temperature: | approx. 900 °F (abt. 482 °C) | ||||||
| Flammability limits in Air: | N/A | ||||||
| Extinguisher Media: | Ferrocerium flints do not burn | ||||||
| Information pertaining to particular dangers for man and environment: | |||||||
| Unusual Fire and Explosion Hazard: Ferrocerium is flammable in powder form as are most metals, i.e. | |||||||
| Aluminum and Magnesium. Ferrocerium in rod form is not flammable and although, in fact, the auto-ignition point is specified by the manufacturer of the Ferrocerium to be 900 °F (abt. 482 °C), | |||||||
| these rods have been subjected to approx. 1700 °F (abt. 926 °C) over a prolonged period of time without flammability or deterioration. | |||||||
| 4. First Aid Measures | |||||||
| Special Instructions : Solid material has no health hazards. | |||||||
| Eye : | May cause irritation due to abrasive action of the material. Flush eyes with warm water for several minutes, occasionally lifting the upper and lower eyelids, If irritation persists, seek medical attention. |
||||||
| Skin : | May cause irritation due to abrasive action of the material. Instantly wash with water and soap and rinse thoroughly. | ||||||
| Remove contaminated clothing and launder. | |||||||
| Inhalation : | Inhalation of powder or dust may cause irritation of respiration tract. If symptoms of pulmonary, remove involved persons from exposure area immediately to fresh air and seek medical attention. | ||||||
| Ingestion : | First aid is not normally required; however, if swallowed and symptoms develop, seek medical attention. | ||||||
| 5. Fire Fighting Measures | |||||||
| Suitable extinguishing agents: Dry sand or salt (NaCl), Class D dry-powder extinguisher. |
|
||||||
| Unsuitable extinguishing agents: Water, CO2-extinguisher and halogenated agents. DO NOT use water. | |||||||
| Personal Protective Equipment: | Wear NIOSH/MSHA approved self-contained breathing apparatus and full protetive clothing. | ||||||
| Specical Procedures : | N/A | ||||||
| Attention : | All exposed surfaces must be covered with sand or metal extinguisher powder ! | ||||||
| Material should not be mixed until the material allowed to cool ! | |||||||
| 6. Accidental Release Measures | |||||||
| Person-related safety precautions: | Wear protective equipment. Keep unprotected persons away. | ||||||
| Measures for environmental protection: Do not allow material to be released to the environment without proper governmental permits. | |||||||
| Measures for cleaning/collecting: | Collect mechanically, small one pick up with shovels, filling in signed closeable containers. | ||||||
| Avoid any contact of material with water and acids. | |||||||
| Attention : | Avoid development of dust and remove all sources of ignition and unburned shavings ! | ||||||
| Rubbing product with metallic objects may cause sparking of fire !!! | |||||||
| 7. Handling and Storage | |||||||
| Handliing : | Use appropriate coatings to protect against the oxidized parts after a certain period. Wash thoroughly after handling. | ||||||
| Storage : | Material should be stored in a cool, clean and dry place with tightly closed containers. | ||||||
| Finely divided particles may be burnable or explosive. | |||||||
| Do not store together with acids. | |||||||
| Store away from oxidizing agents. | |||||||
| Store away from halogens. | |||||||
| Do not store together with alkalis (caustic solutions). | |||||||
| Store away from ignition sources, | |||||||
| Keep container tightly sealed. | |||||||
| Fine powder is highly flammable, when working with powdered material or shavings, use local explosion proof exhaust systems to keep away dust or shavings. | |||||||
| 8. Exposure Controls and Personal Protection | |||||||
| Technical measures for exposure controls : N/A | |||||||
| Limit Values for workplace air: | N/A | ||||||
| Personal protective equipment | |||||||
| Eye Protection : | Protective glasses recommended. | ||||||
| Hand Protection : | Protective gloves recommended, Wash thoroughly after handling. | ||||||
| Respiratory Protection: | Normally not necessary, avoid inhalation of dust. | ||||||
| Body protection : Protective work clothing. | |||||||
| General protective and hygienic measures | |||||||
| The usual precautionary measures should be adhered to in handling the chemicals. | |||||||
| Keep away from foodstuffs, beverages and food. | |||||||
| Instantly remove any soiled and impregnated garments. | |||||||
| Wash hands during breaks and at the end of the work. | |||||||
| 9. Physical/Chemical Characteristics | |||||||
| Physical Form : | Solid cylindrical rod, round bar, cylindrical pellets | ||||||
| Colour : | Metallic dark-grey or in different colours. | ||||||
| Odour : | Odourless | ||||||
| PH value : | N/A | ||||||
| Relative Density : | 6.35 g/cm3 | ||||||
| Information on changes in the physical state : | |||||||
| Melting point : | approx. 2000 °F (abt. 2000 °C) | ||||||
| Boiling point : | approx. 6800 °F (abt. 3760 °C) | ||||||
| Flash point : | N/A | ||||||
| Autoflammability: | Solid bar | approx. 900 °F (abt. 482 °C) | |||||
| Fine powder | approx. 392 °F (abt. 200 °C) | ||||||
| Explosive limits : | N/A | ||||||
| Oxidizing properties: | N/A | ||||||
| Vapour Pressure : | N/A | ||||||
| Solubility : | Water solubility | insoluble | |||||
| Fat solubility | insoluble | ||||||
| 10. Stability and Reactivity | |||||||
| Chemical stability : Stable under normal conditions of storage and handling, but will oxidize in air. | |||||||
| Conditions to avoid : | avoid all possible sources of ignition. air and moisture sensitive. | ||||||
| Incompatible materials: minimize contact with air and moisture; may pontaneously react with air. avoid contact with strong acids, water, air, and oxidizing agents. | |||||||
| Dangerous reactions: | Contact with acids releases flammable gases | ||||||
| Hazardous decomposition products : | contact with strong acids may cause evolution of hydrogen. | ||||||
| 11. Toxicological Information | |||||||
| Toxicological data of material in rod or pellets : N/A | |||||||
| Primary irritant effect on the eye and skin may be caused from ferrocerium powder. | |||||||
| Sensitization : | N/A | ||||||
| Additional toxicological information: | To the best of our knowledge the acute and chronic toxicity of this substance is not fully known. | ||||||
| No classification data on carcinogenic properties of this material is available from the EPA, IARC, NTP, OSHA or ACGIH. | |||||||
| 12. Ecological Information | |||||||
| Do not allow material to be released to the environment without proper governmental permits. | |||||||
| Generally not hazardous for water. | |||||||
| 13. Disposal Considerations | |||||||
| Product: | |||||||
| Recommendation : If possible reuse the unused material, otherwise consult state, local or national regulations for proper disposal. | |||||||
| Hand over to disposers of hazardous waste. Must be specially treated under adherence to official regulations. | |||||||
| Waste disposal method: | for recycling contact manufacturer or supplier. | ||||||
| Uncleaned packagings: | Disposal must be made according to official regulations. | ||||||
| 14. Transport Information | |||||||
| Land transport ADR/RID and GGVS/GGVE (cross-border/domestic) | |||||||
| ADR/RID-GGVS/E Class: None | |||||||
| Maritime transport IMDG/GGVSea: | |||||||
| IMDG/GGVSea Class: None | |||||||
| Air transport ICAO-TI and IATA-DGR: | |||||||
| ICAO/IATA Class: None | |||||||
| Transport/Additional information: | No dangerous goods ! | ||||||
| 15. Regulatory Information | |||||||
| Designation according to EC guidelines: Observe the normal safety regulations when handling chemicals | |||||||
| National regulations: | Observe national regulations on other countries. | ||||||
| Information on the warning label : | None | ||||||
| Information about limitation of use: | For use only by technically qualified individuals. | ||||||
| Water hazard class: Generally not hazardous for water. | |||||||
| 16. Additional Information | |||||||
| The information above is believed to be accurate and represents the best information currently available to SHANGHAI JIANGXI METALS CO., LTD. However, We, SHANGHAI JIANGXI METALS CO., LTD. make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or | |||||||
| damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary | |||||||
| damages, howsoever arising, even if the company has been advised of the possibility of such damages. | |||||||
| Edition Date: | Nov. 26 1995 | FerroCerium flints | |||||